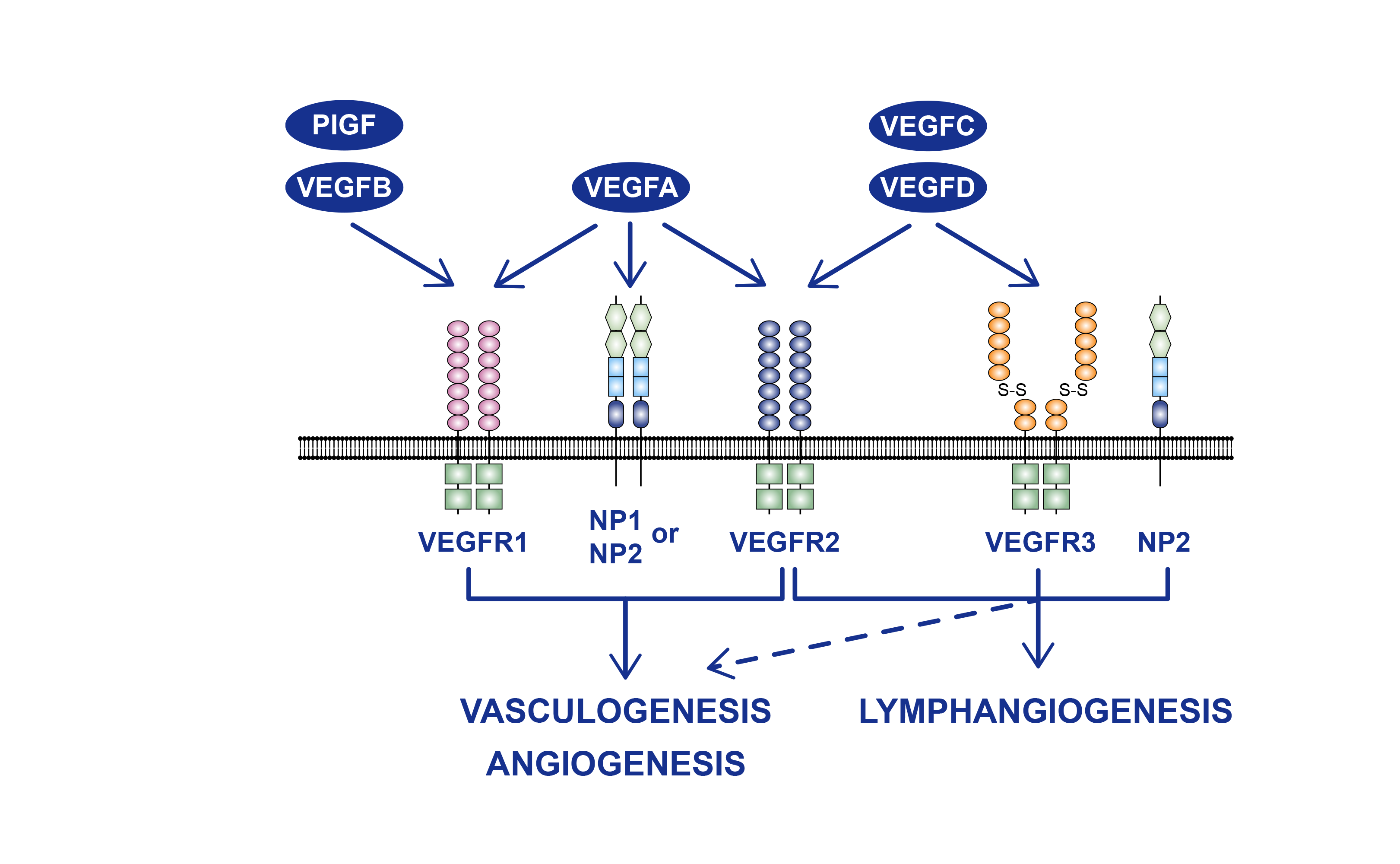
Bevacizumab is a recombinant humanized monoclonal antibody that selectively binds to and blocks the biological activity of human vascular endothelial growth factor (VEGF), reducing tumor angiogenesis and thereby inhibiting tumor growth.MIL60 (bevacizumab) is a biosimilar to bevacizumab. Bevacizumab prodrug Anvitin? was approved by the NMPA on July 9, 2015 as first-line therapy for patients with unresectable advanced, metastatic or recurrent non-squamous NSCLC when used in combination with carboplatin and paclitaxel. Results of a randomized, double-blind, multicenter phase III study comparing the efficacy and safety of MIL60 in combination with paclitaxel and carboplatin to bevacizumab in combination with paclitaxel and carboplatin in subjects with advanced or recurrent non-squamous non-small cell lung cancer (NSCLC) treated initially showed that in the full analysis population (FAS), the ORR12 in the MIL60 and bevacizumab groups were 48.6% and 43.1%. The ORR12 ratio (with stratification factors) assessed by IREC in both groups was 1.14 with a 90% CI of (0.961, 1.322), within a pre-specified equivalence boundary (0.75-1/0.75).
|
|
Secondary endpoint analysis was based on updated data as of October 31, 2020. median DOR was 5.7 months (95% CI 4.5-6.2) for MIL60 and 5.6 months (95% CI 4.3-6.4) for bevacizumab, and median PFS (7.2 months vs. 8.1 months, p=0.9606) and OS (19.3 months vs. 16.3 months, p=0.0755) were not significantly different. Safety Based on updated data as of October 31, 2020, during the combination treatment period of this study, TEAE in the MIL60 and Anvitin? groups (99.6% vs. 98.8%), TEAE associated with MIL60/bevacizumab (79.3% vs. 81.9%), TEAE of grade 3 and above (71.5% vs. 72.6%), grade 3 and above TEAE associated with MIL60/bevacizumab (34.8% vs 39.0%), SAE (28.9% vs 29.0%), SAE associated with MIL60/bevacizumab (15.2% vs 16.2%), TEAE leading to death (2.3% vs 1.9%), and incidence of AESI (48.8% vs 50.2%) were similar between the MIL60 and bevacizumab groups. There was no significant difference in plasma exposure [AUC and peak concentration (Cmax)] between MIL60 and bevacizumab, indicating that MIL60 and bevacizumab have similar characteristics of Pop PK in subjects with advanced or recurrent non-squamous cell NSCLC. During the combination treatment period of this study, one (0.4%) subject in the MIL60 group and one (0.4%) in the bevacizumab group each developed ADA-positive and Nab-negative disease prior to receiving the study drug. In addition, one subject in the bevacizumab group entered the monotherapy phase receiving MIL60 and became ADA-positive at the end-of-treatment visit. Since this subject received both bevacizumab and MIL60 during the study period, it was not possible to confirm which drug the subject developed anti-drug antibodies for. Overall, the incidence of ADA positivity was low. Therefore, it is less likely that MIL60 will have reduced drug efficacy or serious adverse effects due to the development of drug-resistant antibodies. Based on the results of the analysis of the primary efficacy endpoint ORR12, clinical equivalence of northern MIL60 in combination with paclitaxel and carboplatin versus bevacizumab in combination with paclitaxel and carboplatin was demonstrated in subjects with advanced or recurrent non-squamous cell NSCLC in primary treatment.In addition,MIL60 and bevacizumab had similar safety, immunogenicity, and Pop PK profiles. | |